Legislation
As a global company Gurit must comply with chemical regulation present in a number of different regions. A few of these are outlined here. The map below indicates areas of current and developing chemical regulation. It does not include current GHS Regulation.

GHS (Globally Harmonised System)
The Globally Harmonized System of Classification and Labeling of Chemicals or GHS is an internationally agreed-upon system, created by the United Nations. It is designed to replace the various classification and labeling standards used in different countries by using consistent criteria for classification and labeling on a global level. It’s development began at the United Nations Rio Conference in 1992, when the International Labour Organization (ILO), the Organisation for Economic Co-operation and Development (OECD), various governments and other stakeholders met at a United Nations conference. In the EU it is known as CLP (Classification, Labelling and Packaging).
Key points of GHS:
- Global classification system of hazardous chemicals (some variances still remain)
- Harmonised Pictogramms
- Replacement of Risk phrases with Hazard statement
- Replacement of Safety phrases with Precautionary statement
- Globally standardised SDS (Safety Datasheet) Format
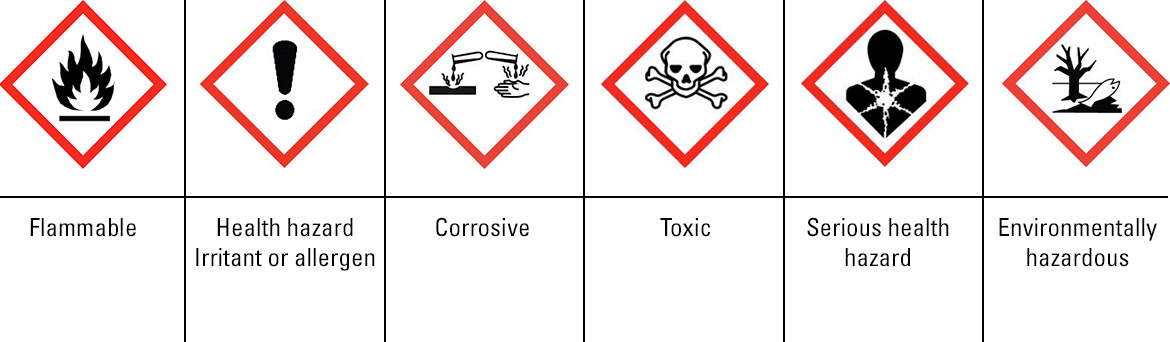
Implementation timescales vary by region meaning the appropriate labelling and MSDS (material safety datasheet) must be provided for the country for which the product is sent to in a format required by that region.
REACH – Registration, Evaluation, and Authorisation of Chemical
On the 1st June 2007 the largest piece of legislation ever to emerge from the European Union was introduced entitled REACH (Registration, Evaluation and Authorisation of Chemicals), EC 1907/2006.
REACH is an acronym for Registration, Evaluation and Authorisation of Chemicals and requires the registration of all chemicals manufactured in or imported into the EU in volumes greater than 1t/annum by 1st June 2018.
The purpose of REACH is:
- To provide a high level of protection of human health and the environment from the use of chemical.
- To make the people who place chemicals on the market (manufacturers and importers) responsible for understanding and managing the risks associated with their use.
- To allow the free movement of substances on the EU market.
- To enhance innovation in and the competitiveness of the EU chemicals industry.
- To promote the use of alternative methods for the assessment of the hazardous properties of substances e.g. quantitative structure-activity relationships (QSAR) and read across.
As a downstream user of chemicals ourselves it is important that we manage the introduction of REACH to protect our supply chain and continue to manufacture products of the highest quality and consistency.
Toxic Substances Control Act (TSCA)
The Toxic Substances Control Act (TSCA) is a United States law, passed in 1976 and administered by the United States Environmental Protection Agency (EPA). TSCA is an inventory of chemicals currently in use on the US market. Chemicals listed on the TSCA inventory are referred to as “existing chemicals”, while chemicals not listed are referred to as new chemicals. It prohibits the manufacture or importation of chemicals that are not on the Inventory or subject to one of many exemptions.
The introduction of new or already existing chemicals is regulated by the TSCA three main objektives:
- assess and regulate new commercial chemicals before they enter the market,
- to regulate existing chemicals that pose an “unreasonable risk to health or to the environment”
- regulate the distribution and use of these chemical
The EPA reviews new chemical notifications and if it finds an “unreasonable risk to human health or the environment,” it may regulate the substance by limiting uses, production volume or even banning it.
In 2016, the Frank R. Lautenberg Chemical Safety for the 21st Century Act (TSCA reform) was passed as the first major overhaul in many years. Part of the reform has been the TSCA inventory ‘reset’. This is a program devised to overhaul the TSCA inventory and designate chemicals into either active or inaktive.
The following pages give more information on the various areas of product stewardship and regulatory compliance. Any questions on product stewardship can be sent to: regulatory@gurit.com

